The Patient Perspective: Evaluating Time and Treatment While Living with Metastatic Breast Cancer
Time is a precious resource for patients living with ER+, HER2- metastatic breast cancer (mBC). Those living with ER+, HER2- mBC are dealing with an incurable disease. However, with effective treatments available, patients may live for many years. Treatment can delay mBC from progressing and allow patients invaluable time to spend with friends and family as well as on activities that are meaningful to them. The time commitment of a treatment may be an important concern patients weigh when considering treatment options, like oral medications or injections. In fact, in a testament to how much time patients spend in care, a new study by Ipsos finds that these ER+, HER2- mBC patients commit, on average, over two hours for a typical appointment with their oncologist.
There are several approaches for the treatment of breast cancer and treatment options will vary based on stage and tumor characteristics. In ER+, HER2- mBC, potential treatment options for patients include chemotherapy, endocrine therapy (e.g., tamoxifen, aromatase inhibitors (AI), and selective estrogen receptor down-regulators (SERDs)), and therapies that target different pathways (e.g., CDK4/6, PI3K, AKT and mTOR inhibitors)1. Treatment selection can be determined by a number of factors, such as extent of disease, presence of tumor biomarkers, patient clinical status, or patient preference2,3,4. In instances where a patient progresses after receiving an AI, a SERD is a common class of treatment used – alone or in combination with other treatments5,6. SERDs have proven efficacy in ER+, HER2- mBC and have commonly been administered as an intramuscular (IM) injection in a hospital or clinic setting; however, an oral formulation is also available2,4,5,6,7. They are also recommended by treatment guidelines as an option for certain patients with ER+, HER2- mBC4. Research conducted by Ipsos in collaboration with Living Beyond Breast Cancer and funded by Eli Lilly and Company specifically surveyed patients living with ER+, HER2- mBC to understand their attitudes and experiences.
Based on this research, Ipsos finds that the time spent at the oncologist’s office is a burden for most ER+, HER2- mBC patients surveyed, and most of these patients would prefer taking oral medications at home over traveling to a doctor’s office and receiving an IM injection. These findings highlight the importance of understanding the lives and perspectives of mBC patients and considering their support networks as patients live with an uncertain future.
Patient perceptions and experience with IM injections
While IM injections can be an important and valuable part of care plans for those with ER+, HER2- mBC, many of these patients report and perceive some downsides of this form of care, like painful side effects and the time-consuming nature of attending oncologist appointments. Specifically, 43% of ER+, HER2- mBC patients surveyed feel that the pain of IM injections would be a drawback to this type of treatment. A similar proportion of these patients (44%) perceive that anxiety about getting an injection would be a drawback in and of itself. Forty-seven percent of these patients report that injection site soreness and the impact it would have on their daily lives would be a drawback of IM injections.
Being able to avoid this injection-specific pain is appealing to most of the ER+, HER2- mBC patients Ipsos surveyed. A majority (62%) feel that not having to experience pain and soreness from an injection is a benefit of an oral mBC treatment. However, oral medications may have associated side effects for patients, too.
Patient concerns about the time commitment for IM injections
ER+, HER2- mBC patients cite how time-consuming it is to go to medical facilities as a disadvantage of treatment that uses IM injections. Fifty-two percent of these patients feel that a drawback of IM injections is the time needed to travel to the oncologist’s office. In fact, 35% of these patients say they need to take paid time off (PTO) or unpaid time from work to accommodate trips to the oncologist. The time and preparation for these treatments can impact others in addition to the patient. Thirty-three percent of these ER+, HER2- mBC patients need loved ones to accompany them to appointments with their oncologist, and 22% need to cancel or reschedule family activities to attend them.
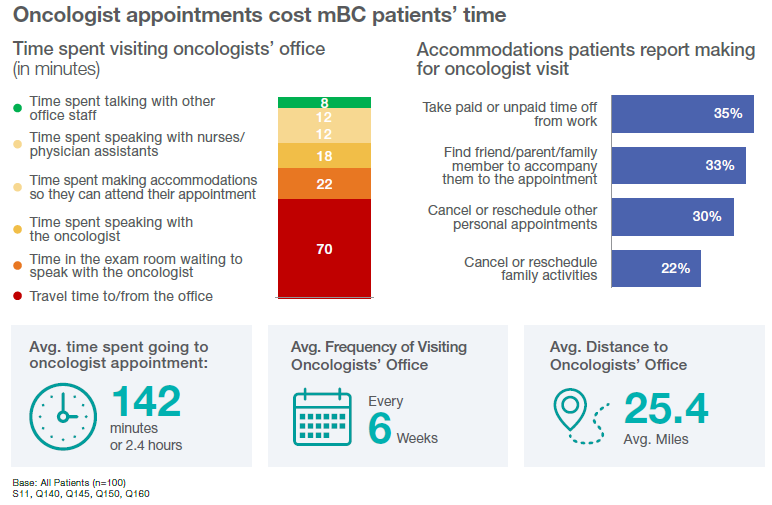
This isn’t a marginal time commitment. On average, it takes just under two and a half hours for these patients to attend an appointment at their oncologist’s office, which includes traveling, waiting, and discussing treatment with office staff. Patients report an average of going to these appointments every six weeks and traveling roughly 25 miles, meaning treatment that involves monthly IM injection might add to the regular and consistent burden that’s already on patients and their support networks.
Perceived benefits of oral medications according to patients
Given this time commitment, many ER+, HER2- mBC patients that Ipsos surveyed perceive the lack of travel as a benefit of oral treatments. When asked about the perceived benefits of oral breast cancer treatments, 51% of ER+, HER2- mBC patients report that an oral treatment would give them more time to themselves to do the activities they love. To that end, 77% of ER+, HER2- mBC patients say they would prefer taking an oral medicine daily instead of traveling to their oncologist’s office to receive an IM injection.
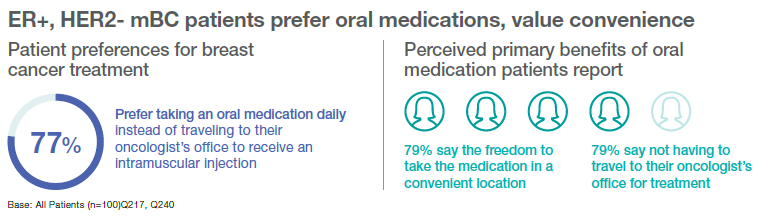
An oral option also gives them a sense of control over their lives; seventy-five percent of ER+, HER2- mBC patients believe taking a daily oral medication makes them “feel like they are actively fighting cancer in their lives every day.” A similar proportion of these patients (73%) also feel that oral medication would not affect their daily routine. These ER+, HER2- mBC patients see the benefits of oral medications, saving them time and money. Sixty-one percent say taking oral therapy at home would ease the financial burden associated with traveling to the doctor’s office. Even more, 79%, view the freedom to take the medication in a convenient location and eliminating the need for travel as the primary benefits of oral medications. These benefits weigh heavily for these patients when evaluating potential breast cancer treatments. When asked to assess the top qualities of potential breast cancer medications, 79% of ER+, HER2- mBC patients consider whether the medication’s side effects are tolerable. An equivalent proportion (79%) considers how medications impact their quality of life. After that, at 33%, the next biggest concern is out-of-pocket cost. Overall, when considering breast cancer treatments, more ER+, HER2- mBC patients value tolerable side effects and control over how they spend their time over cost.
Conclusion
The results of this research demonstrate the time-burden on ER+, HER2- mBC patients and their loved ones when going to receive care and the potential difficulty of care when an IM injection is part of their treatment plan. Metastatic breast cancer patients face an uncertain future. Painful side effects and time spent to access treatment are a burden to patients and their support networks. Time saved by reducing trips to appointments is an important part of how these patients weigh and evaluate their care plans. Patients value oral options and appreciate the freedom these medications can give them, as a way to better use their precious time doing activities that are meaningful. Through this research, Ipsos amplifies ER+, HER2- mBC patients’ preference towards oral medications as a viable and preferred mode of administration that ease some of the burdens IM injections can have on their time, body, and mind.
Methodology
These are some of the findings of Ipsos research conducted in collaboration with Living Beyond Breast Cancer and funded by Eli Lilly and Company. This research program included a primary quantitative survey component, followed by a qualitative social listening exercise. The survey was conducted between August 23-October 18, 2023. For this survey, a sample of n=100 women with estrogen receptor positive (ER+), HER2-negative metastatic breast cancer between the ages of 35-75 from the continental U.S, Alaska, and Hawaii were interviewed online in English. The sample was randomly drawn from Ipsos’ online panel partners. To qualify for the survey, respondents needed to be currently receiving systemic treatment for their breast cancer under an oncologist’s care but have no prior usage of the intramuscular injection fulvestrant, in addition to the sample qualifications listed above.
A Social Intelligence component was conducted by mining data from ER+, HER2- metastatic breast cancer patients with experience on fulvestrant from social, forum and mainstream media sources using Ipsos’ Synthesio as well as search services Google Trends and Answer the Public from March 12th, 2022—March 13th, 2023. The nature of that important research was not explored in this paper.
Please follow this link for the full survey results and methodological information about the survey and the Social Intelligence research. Or revisit our on demand deep dive webinar here.
Sources
- Huppert L, Gumusay O, Idossa D, Rugo H. Systemic therapy for hormone receptor-positive ... - wiley online library. March 20, 2023. Accessed January 2024. https://acsjournals.onlinelibrary.wiley.com/doi/10.3322/caac.21777.
- Li J, Wang Z, Shao Z. Fulvestrant in the treatment of hormone receptor-positive/human epidermal growth factor receptor 2-negative advanced breast cancer: A Review. Fulvestrant in the treatment of hormone receptor-positive/human epidermal growth factor receptor 2-negative advanced breast cancer: A review. May 2019. Accessed January 2024. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6536994/.
- Mosele F, Stefanovska B, Lusque A, et al. Outcome and molecular landscapeof patients with PIK3CA-mutated metastatic breast cancer. January 24, 2020. Accessed February 7, 2024. https://www.annalsofoncology.org/article/S0923-7534(19)39094-5/fulltext.
- Burstein HJ, DeMichele A, Somerfield MR, et al: Testing for ESR1 mutations to guide therapy for HR-positive, human epidermal growth factor receptor 2-negative metastatic breast cancer: ASCO guideline rapid recommendation update. May 17, 2023. Accessed February 2024. https://ascopubs.org/doi/10.1200/JCO.23.00638.
- Sammons S, Kornblum NS, Blackwell KL. Fulvestrant-based combination therapy for second-line treatment of hormone receptor-positive advanced breast cancer. Fulvestrant-Based Combination Therapy for Second-Line Treatment of Hormone Receptor-Positive Advanced Breast Cancer. February 2019. Accessed January 2024. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6407749/.
- Boér K. Fulvestrant in advanced breast cancer: Evidence to date and place in therapy. Fulvestrant in advanced breast cancer: evidence to date and place in therapy. July 2017. Accessed January 2024. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5502950/.
- Fulvestrant injection. Cleveland Clinic. Accessed January 2024. https://myclevelandclinic.org/health/drugs/18313-fulvestrant-injection.

![[WEBINAR] 2026 KEYS: Battle for Attention](/sites/default/files/styles/list_item_image/public/ct/event/2026-02/thumbnail-keys-Battle-Attention_0.jpg?itok=ftV-emtI)

![[WEBINAR] How to build trust in the AI era](/sites/default/files/styles/list_item_image/public/ct/publication/2026-02/thumbnail-trust-ai_0.jpg?itok=n6Xc78CU)