A New Era for Pharmaceutical Advertising
KEY TAKEAWAYS:
- New rules on direct-to-consumer pharmaceutical advertising require more prominent disclosure of drug side effects
- Brands concerned about complying with the new rules should avoid shifting fully to generic disease-state awareness campaigns, which tend to be less effective with consumers
- Pharmaceutical companies should consider this an opportunity to think more ambitiously about how creativity can deliver distinctiveness in a category that can often suffer from a “sea of sameness”
The pharmaceutical industry is undergoing a transformative shift in the landscape of direct-to-consumer (DTC) advertising, with new federal government regulations requiring even more prominent disclosures of side effects. Here’s what to know and what you should do.
The FDA’s new regulations now require prescription drug advertisements on television or radio to “present the major statement relating to side effects and contraindications in a clear, conspicuous, and neutral manner.”
To date, fair-balance communication, which lists side effects and contraindications of the drug advertised, is often presented quickly and visually minimized, using fast-paced narration and small text potentially hindering viewers’ comprehension. This generally occurs in the middle section of the ad, enabling the brand to close the ad with a final branded message.
The new regulations are designed to enable greater transparency on the risks associated with the advertised drugs. Some may view this change as a risk or challenge and may consider allocating their resources to shift to a heavier mix of unbranded disease-state awareness initiatives from branded DTC.
However, Ipsos believes that branded DTC remains a critical component for driving brand growth and should continue to be a key consideration in marketing campaigns.
Our data show that up to now, branded ads significantly outperform disease-state awareness campaigns across several key metrics, including relevance to the target audience, information value, and the ability to provide new information, plus have a direct link to the brand being advertised. While there are certain market situations that may favor one type of ad over the other, this makes a compelling reason to include DTC in your campaign media mix.
At Ipsos, we see the new fair-balance regulations as a potential opportunity to re-think creativity for DTC pharmaceutical advertising that can lead to even stronger effectiveness.
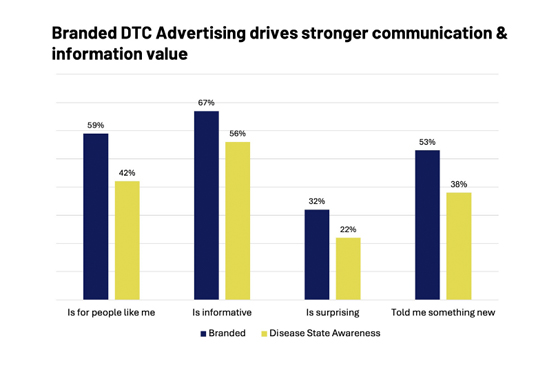
DTC Pharmaceutical Ads Gain Attention In Unique Ways
Ipsos’ measure of Brand Attention – the ability of an ad to capture attention and be correctly linked to the brand - is one key component to in-market success. Historically, DTC Pharmaceutical ads have been effective in gaining attention, as they are testing among their intended target groups:
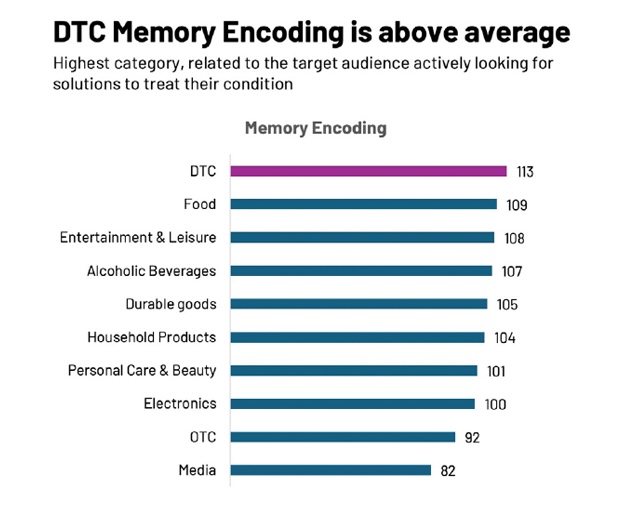
Source: Ipsos Creative|Spark US database
Branding is a Key Challenge
However, their challenge has been their ability to link the creative to the brand:
By prioritizing creative quality, leveraging the power of branded advertising, and listening to the voice of the target patients, Ipsos believes that pharmaceutical companies can continue to effectively reach and engage their target audience, fostering brand growth and success in this new era of advertising.
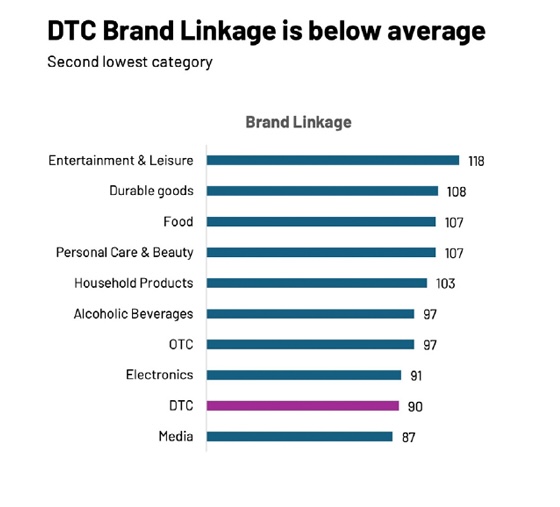
Source: Ipsos Creative|Spark US database
Branding in this category is often challenging because of brand names that are difficult to pronounce and not intrinsically meaningful. Creative techniques such as jingles, logos, music and brand mnemonics – amongst others – are often used to try to overcome that issue, yet branding for this category advertising is still not fully effective, on average.
The creative approach is also a factor to consider. Ipsos’s database shows that 79% of pharmaceutical ads tested are slice-of-life/vignette style, making them largely undifferentiated among brands – regardless of therapeutic area or drug. This over-reliance on a single creative approach has led to what some see as formulaic, which makes it difficult for pharmaceutical brands, on average, to stand out.
The Misfits Framework is an analysis Ipsos has done to demonstrate how creativity in advertising sparks brand growth derived from extensive meta-learnings. This framework reveals that ads with the highest probability of success offer a strong creative experience, lead with innovative ideas that shed new light on the brand, and forge meaningful connections with people.
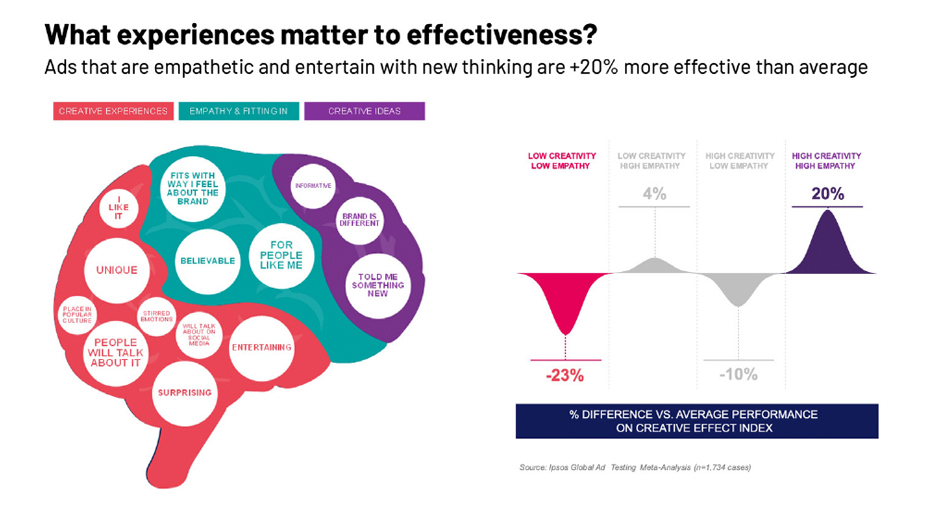
While DTC advertising tends to be high on empathy and creative ideas, they skew lower on delivering creative experiences, which speaks to the “sea of sameness” pharmaceutical ads typically have, given the high proportion leveraging slice-of-life/vignette approaches.
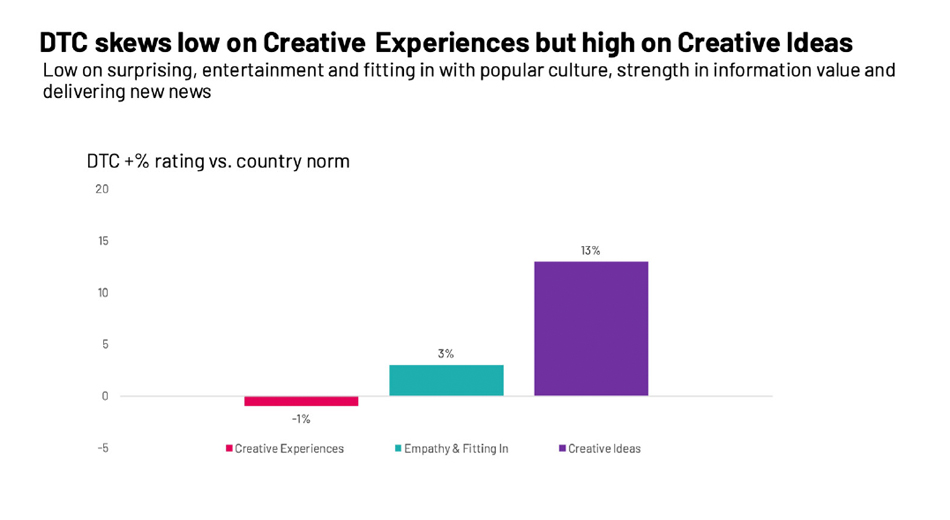
Since we know creative experiences are a key driver of Brand Attention, exploring different ways to drive those creative experiences will be key to help further differentiate and build your brand.
Moving forward, this raises some potential questions.
- Does this change in fair-balance communication bring an opportunity to think more ambitiously about how creativity can deliver distinctiveness in this category?
- What opportunities exist to potentially enhance overall engagement if fair-balance may no longer be part of the creative component?
- Do you try and re-gain attention following the fair-balance, or do you treat fair-balance as the end of the ad?
- Do you “own the fair-balance” and brand it?
- How do you ensure your distinctive brand assets are integrated into the most engaging pieces of your story?
It is essential to develop advertising that is not only compliant with the new regulations but also resonates with audiences on a deep, emotional level, allowing pharmaceutical brands to continue playing a vital role in educating and empowering patients to make informed decisions about their health.
While the new FDA rules for DTC pharmaceutical advertising present challenges, they also offer a unique opportunity for brands to re-evaluate their strategies, focus on creative excellence, and forge stronger connections with their target audiences.
Ipsos, with its vast repository of advertising effectiveness data and experts, deep industry knowledge, and specialist qualitative experts, possesses a deep understanding of the pivotal role that creative quality plays in driving impact in healthcare advertising.
To ensure that your brand is putting forth the most impactful advertising possible, let Ipsos help you:
- Test ads in a safe space. No need to put your full marketing budgets behind ads with untested new treatments! We specialize in finding hard-to-reach target groups for rare disease indications and can apply leading predictive metrics to the test results.
- Explore different fair-balance demo treatments. Easily compare like-for-like replacements of demos within the context of the broader creative idea. Qualitative and Quantitative research both help to uncover the nuanced messaging different treatments can bring.
- Compare new ads to past creative. Use validated predictive KPIs to predict the potential change in effectiveness of the new campaign. Know before you launch so there are no surprises.
- Understand the relative strengths and weaknesses of Branded vs. Disease State Awareness ads. Don’t change to an unbranded disease awareness campaign lightly. With a rich history of testing both, we can put the relative paths in better context before you launch.
By prioritizing creative quality, leveraging the power of branded advertising, and listening to the voice of the target patients, Ipsos believes that pharmaceutical companies can continue to effectively reach and engage their target audience, fostering brand growth and success in this new era of advertising.



![[WEBINAR] The Super Bowl’s Best Ads of 2025](/sites/default/files/styles/list_item_image/public/ct/event/2025-01/linkedin_1.png?itok=LXfnrrAL)